Speakers
Here you can find a brief presentation of our speakers and an abstract of the work they will present during the event.
The speakers are listed below, ordered based on their presentation slot.
Prof. Dr. Gert-Jan M. Gruter

Gert-Jan has a background in Polyolefin catalysis (DSM; now Sabic) and has been Professor of Polymer Catalysis at Eindhoven University of Technology (1999-2006). After joining Avantium in 2000, he was the CTO, responsible for developing Avantium’s technology portfolio:
- Furan dicarboxylic Acid (FDCA; start-up commercial plant) for 100% bio-based PEF polyester (bottles, fibers and film)
- “DAWN Biorefinery Technology” for the production of pure glucose (in Pilot Plant), now with focus on waste textiles as feedstock.
- “Volta Technology” for the electrochemical reduction of CO2 to polyester monomers (oxalic- and glycolic acid) and their polymers.
Gert-Jan is an inventor on more than 100 PCT patent families; he was elected “2014 European CTO of the year” and runner-up European inventor of the year in 2017 for FDCA/PEF. Gert-Jan is currently also professor Industrial Sustainable Chemistry at the University of Amsterdam (UvA), where he is also working on the plastic transition (high Tg copolyesters for ABS replacement (with LEGO) and for re-use; biodegradable polyesters; chemical recycling and upcycling with a focus on waste textiles (cotton/ polyester blends); consumer psychology; PET micro plastics quantification).
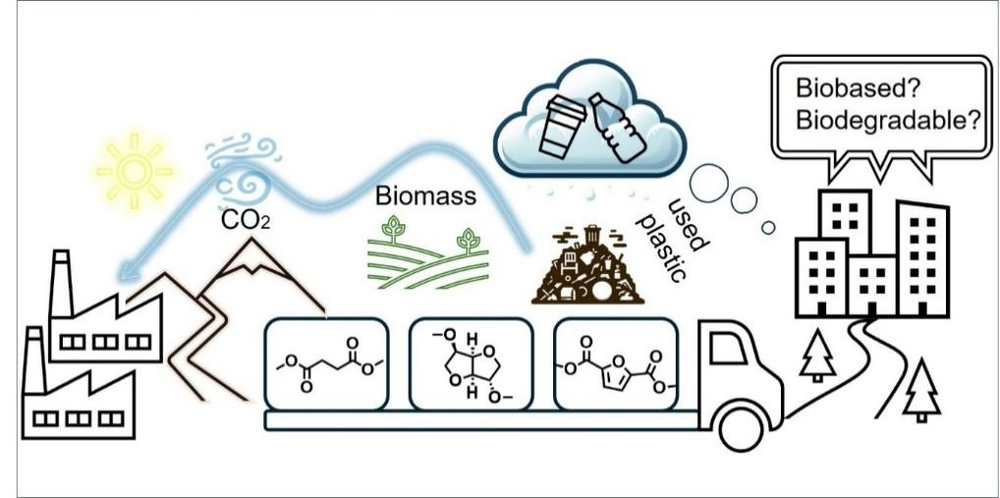
Abstract: The future of plastics from biomass and CO2 feedstock
The combination of the predicted polymer market growth, the poor recyclability of polyolefins and the emergence of renewable feedstocks creates a fantastic opportunity for sustainable polymers. To replace fossil-based feedstock, there are only three sustainable alternatives: biomass, CO2, and existing plastics (via mechanical and/or chemical recycling).
This lecture will focus on future polymer options with the highest techno-economic potential: why the transition to new feedstocks (biomass and CO2) will /should lead to a transition of materials (from polyolefins to polycondensates (polyesters)) and why this is not a threat but an opportunity.
Dr. Ir. Tom Sleutels

Tom Sleutels graduated from Wageningen University with a bachelor’s and master’s degree in Bioprocess Engineering. He received his PhD in Environmental Technology after conducting research in collaboration with Wetsus, the European Centre of Excellence for Sustainable Water Technology. It is here that he continued his research and became a scientific staff member. Since 2021, he has been affiliated with the University of Groningen, where he is an associate professor in the group that develops products and processes for Biotechnology.
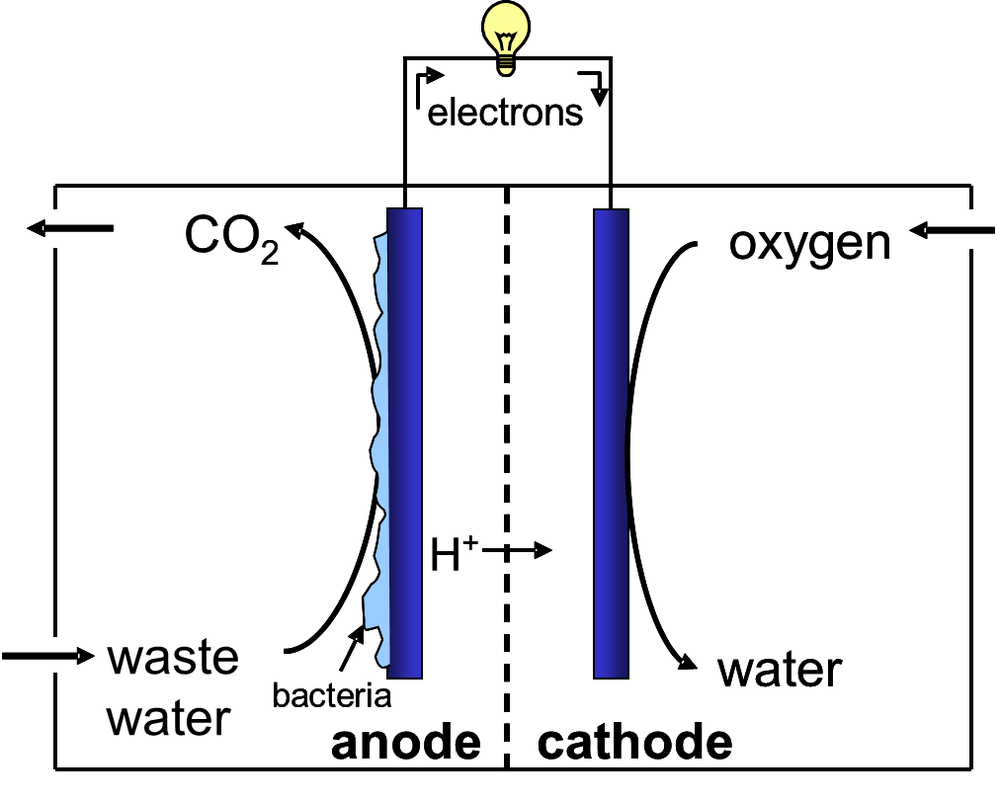
Abstract: Microbial Electrochemical Systems for Waste Water Treatment
For over a century, municipal wastewater treatment plants have aided in increasing sanitation in especially urban areas, as they treat the collected sewage. Besides an extensive and expensive piping network, this requires large amounts of energy and chemicals. Nowadays, these facilities are considered a great opportunity for the recovery of energy, carbon and nutrients. Microbial Electrochemical Systems are an emerging technology that is able to recover energy from organic waste material. This technology makes use of electrochemically active microorganisms that grow and live on an electrode and are able to exchange electrons with this conductive material. This way, they can produce an electrical current from wastewater. Besides, Microbial electrochemical technologies offer the opportunity to produce value-added compounds from carbon dioxide with only the input of renewable electricity. ù
In this presentation, I will introduce electroactive microorganisms and how they are used in Microbial Electrochemical Technologies and how they can help in changing our wastewater treatment facilities into energy, nutrient and chemical producing factories.
Prof. Dr. Michael Lerch
Michael M. Lerch is an assistant professor at the University of Groningen (the Netherlands), working on self-regulated soft materials and soft robotics (www.lerchlab.com).
He holds an MSc in Interdisciplinary Sciences from ETH Zurich and a PhD in supramolecular and photochemistry based on his work on ‘Donor–Acceptor Stenhouse Adducts’ with Prof. Feringa.
During a NWO Rubicon postdoctoral fellowship in the Aizenberg group at the WYSS Institute and Harvard University, he developed microstructured liquid crystalline elastomer surfaces with self-regulated motion and feedback-controlled optically active hydrogels. His research focuses on chemically controlled soft robotics and smart coatings to create a more sustainable and equitable future.

Abstract: Sustainable coatings – Fundamental Science meets Industrial Relevance

Coatings are everywhere around us, from plastic coffee cups and waterproof jackets to cars and airplanes. Coatings protect underlying materials, make things aesthetically pleasing, and may even add additional functionalities such as self-cleaning or self-healing. Yet coatings can be resource-intensive, even toxic, and cause issues for the recycling process. Novel solutions for reducing coatings’ environmental footprint are urgently needed, both with regard to what they are made from (i.e., bio-feedstocks) and to what functions they have that increase life-time and recyclability. In this presentation, I will give an introduction to how coatings work, why they come with problems, and some approaches our research group is taking (in collaboration with industry) to make better and more sustainable coatings.
Alexandra Matei

Alexandra was born in Bucharest, Romania. She obtained her BA in Biochemistry and Molecular Biology from Bryn Mawr College (Pennsylvania, United States) in 2020. After her bachelor's, Alexandra moved to Groningen for her MSc in Chemistry: Green Chemistry and Catalysis Track.
She conducted her Master's thesis project in the Feringa group, working on the regioselective preparation of 1,5-enynes from in situ generated propargyllithium species. After completing her MSc in 2022, Alexandra returned to the Feringa group to start her PhD focused on developing photo- and electrochemical methodologies for the direct oxidation of terminal alkenes.
In 2024, Alexandra joined the core Green Labs team at the FSE, and since then, she has been the chair of the waste subgroup, supporting the faculty initiatives to improve sustainability aspects around waste management.
Abstract: Implementing sustainable lab practices at the Faculty of Science and Engineering
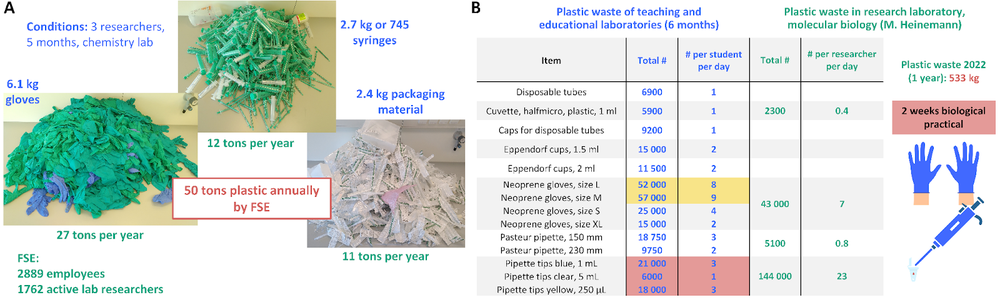
Globally, laboratory research generates an estimated 5.5 million tonnes of plastic waste annually, accounting for approximately 2% of total global plastic waste.1 Common types of plastics used in labs include polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET), polymethyl methacrylate (PMMA), and polystyrene (PS). Laboratory consumables contributing to this waste range from pipette tips, tubes, and filter bottles to gloves, weighing boats, well plates, and packaging materials.
At the University of Groningen, all laboratories within the Faculty of Science and Engineering (FSE) focus on STEM research. In early 2022, the Green Labs team conducted a five-month case study in a chemistry lab, where plastic waste was collected and analysed from three representative researchers. The study found that in this period, 6.1 kg of glove waste, 2.7 kg of syringe waste, and 2.5 kg of packaging waste were produced. When extrapolated to 1,762 active lab researchers within FSE, this amounts to approximately 9 tons of glove waste, 4 tons of syringe waste, and 4 tons of packaging waste annually.2 These findings underscore the urgent need for more sustainable and circular solutions in laboratory consumables.
The Green Labs team is not only supporting different frameworks for running research laboratories more sustainably, but is also pioneering protocols for recycling traditionally “one-time-use” consumables from biology laboratories together with relevant stakeholders such as the health and safety department and waste management companies. This talk will highlight current efforts and the progress of establishing such protocols faculty-wide.
Prof. Dr. Sahar El Aidy
El Aidy's academic journey spans continents, from studying Pharmaceutical Sciences in
Alexandria (Egypt) to her MSc and PhD in Microbiology in Wageningen, the Netherlands.
Following postdoctoral research at University College Cork (Ireland), she held positions
including a Rosalind Franklin Fellow and Associate Professor at the University of Groningen.
Since April 2024, she has been appointed as Professor and Chair of Microbiome Engineering at the University of Amsterdam, NL.
El Aidy secured various research grants, including from NWO and the Parkinson Fonds, and engaged in multiple industrial collaborations. Recognised for her achievements, she was appointed to the Young Academy Groningen and received the prestigious Athena Award from NWO. Her contributions extend to diverse activities, including regular contributions to newspapers and magazines, participation in health science channels and professional outreach programs, delivering public lectures, and being involved in impactful projects such as a commissioned film exploring the microbiome's impact on mental health.

Abstract: The Chemical Dialogue Between Gut Microbes, Food, and Medicine
Our gut microbiota are master chemists. They transform nutrients, drugs, and host molecules into a vast array of metabolites that can alter physiology, behavior, and disease trajectories. My research explores this chemical dialogue at the interface of microbiology, chemistry, and human health. By integrating analytical chemistry, microbiology, ecological modeling, and physiology, my lab has uncovered how gut microbes metabolize not only levodopa, the main treatment for Parkinson’s disease, but also serotonin precursors, anti-inflammatory peptides, and dietary polyphenols. These microbial transformations influence drug effectiveness, reshape microbial communities, and feed back onto intestinal motility, a key regulator of the gut–brain axis. Beyond individual examples, our work reveals fundamental ecological principles of microbial adaptation, cooperation, and competition, offering new frameworks to understand the microbiome “health” as a dynamic, evolving property. Through synthetic communities and gut models, we investigate how functional interactions and chemical signaling, rather than just microbial composition, determine clinical outcomes and resilience. In this lecture, I will show how gut bacteria-driven chemistry connects food, microbes, drugs, and the brain, and how going “back to base” in understanding these interactions can inspire new strategies for microbiota-based therapies and precision medicine.





